Human Whole Blood mQTL Atlas (CRIC)
Systematic integrated analysis of genetic and epigenetic varition in diabetic kidney disease
Welcome to the Susztaklab human blood mQTL atlas
Poor metabolic control and host genetic predisposition are critical for diabetic kidney disease (DKD) development. The epigenome integrates information from sequence variations and metabolic alterations. Here, we performed a genome wide methylome association analysis in 500 subjects with DKD from the Chronic Renal Insufficiency Cohort for DKD phenotypes, including glycemic control, albuminuria, kidney function and kidney function decline. We show distinct methylation patterns associated with each phenotype. We define methylation variations that are associated with underlying nucleotide variation (methylation quantitative trait loci; mQTL) and show that underlying genetic variations are important drivers of methylation changes. We implemented Bayesian multi-trait co-localization analysis (moloc) and summary data-based Mendelian randomization (SMR) to systematically annotate genomic regions that show association with kidney function, methylation and gene expression. We prioritized 40 loci, where methylation and gene expression changes likely mediate the genotype effect on kidney disease development. Functional annotation suggested the role of inflammation, specifically, apoptotic cell clearance and complement activation in kidney disease development. Our study defines methylation changes associated with DKD phenotypes, the key role of underlying genetic variations driving methylation variation and prioritizes methylome and gene expression changes that likely mediate the genotype effect on kidney disease pathogenesis.
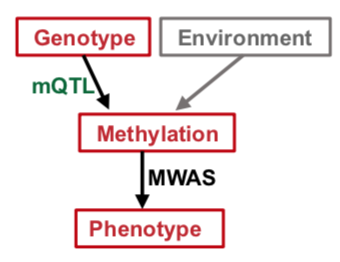
 mQTLs identified in about 500 human blood samples with diabetic kidney disease from the Chronic Renal Insufficiency Cohort are available online.
mQTLs identified in about 500 human blood samples with diabetic kidney disease from the Chronic Renal Insufficiency Cohort are available online.